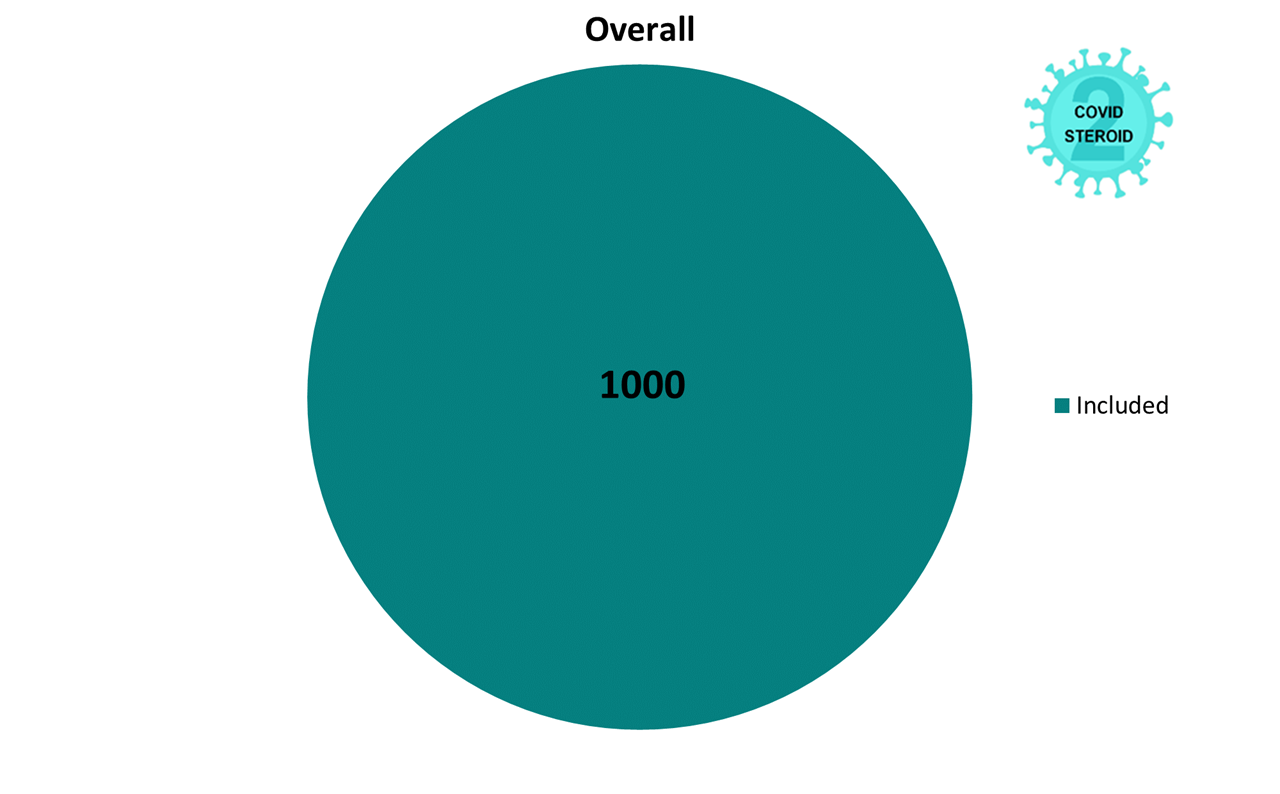
The COVID STEROID 2 trial assessed the effects of a higher (12 mg) compared to (6 mg) lower dose of dexamethasone for patients with COVID-19 and severe oxygen deficiency.
Results
- We assessed the effects of a higher dose of dexamethasone on the number of days alive without life support, the number of days alive and out of hospital, mortality, or serious adverse reactions after 90 days and mortality or health-related quality of life after 180 days.
- The results demonstrated possible benefit from the higher dose of dexamethasone.
The results have provided important knowledge regarding corticosteroid treatment for COVID-19 patients and will benefit future COVID-19 patients. The main results have been published in the two separate publications listed below.
Publications
- COVID STEROID 2 Trial Group. Effect of 12 mg vs 6 mg of Dexamethasone on the Number of Days Alive Without Life Support in Adults With COVID-19 and Severe Hypoxemia: The COVID STEROID 2 Randomized Trial. JAMA 2021; 326: 1807-17.
- Granholm A et al. Long-term outcomes of dexamethasone 12 mg versus 6 mg in patients with COVID-19 and severe hypoxaemia. Intensive Care Med 2022; 48: 580-89.
Trial Sponsor
Professor, MD, Anders Perner
University Hospital Copenhagen, Rigshospitalet
anders.perner@regionh.dk

Useful Documents

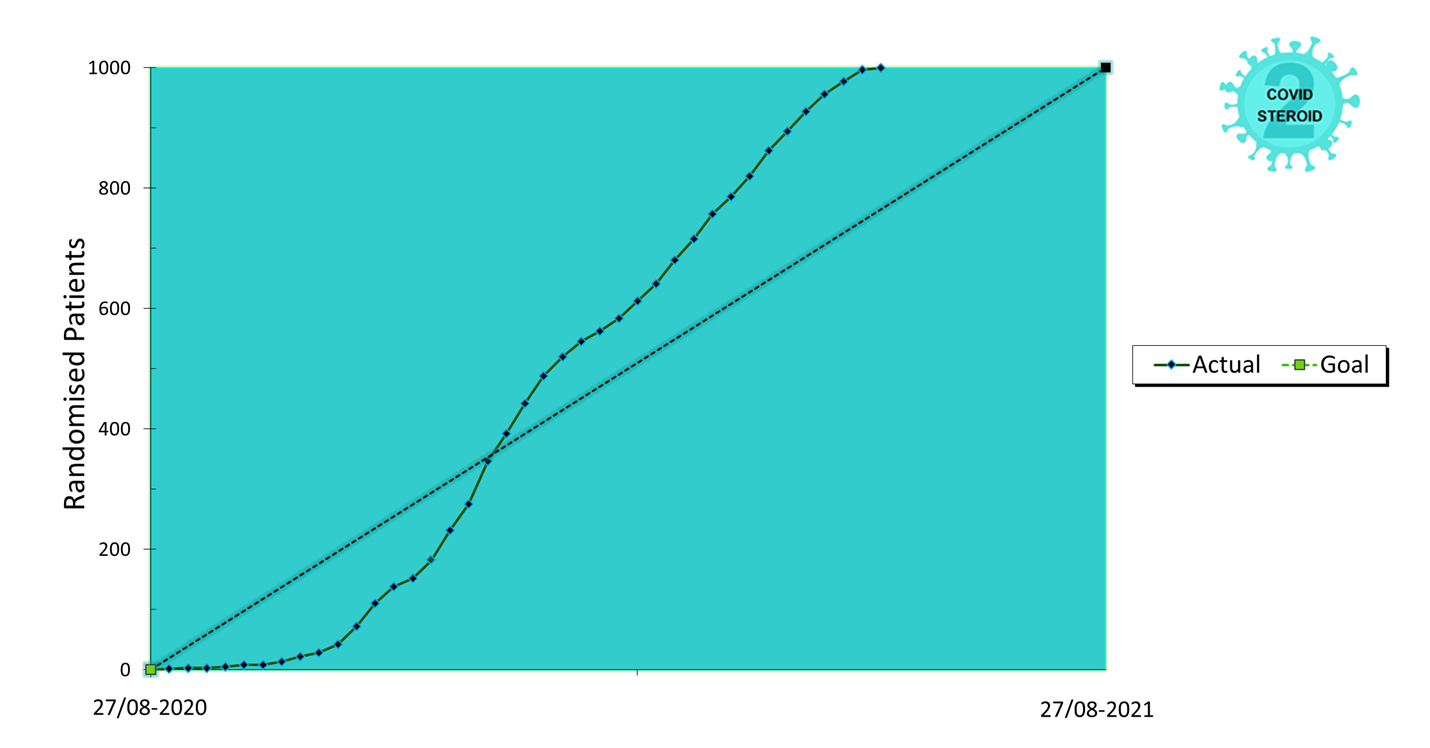
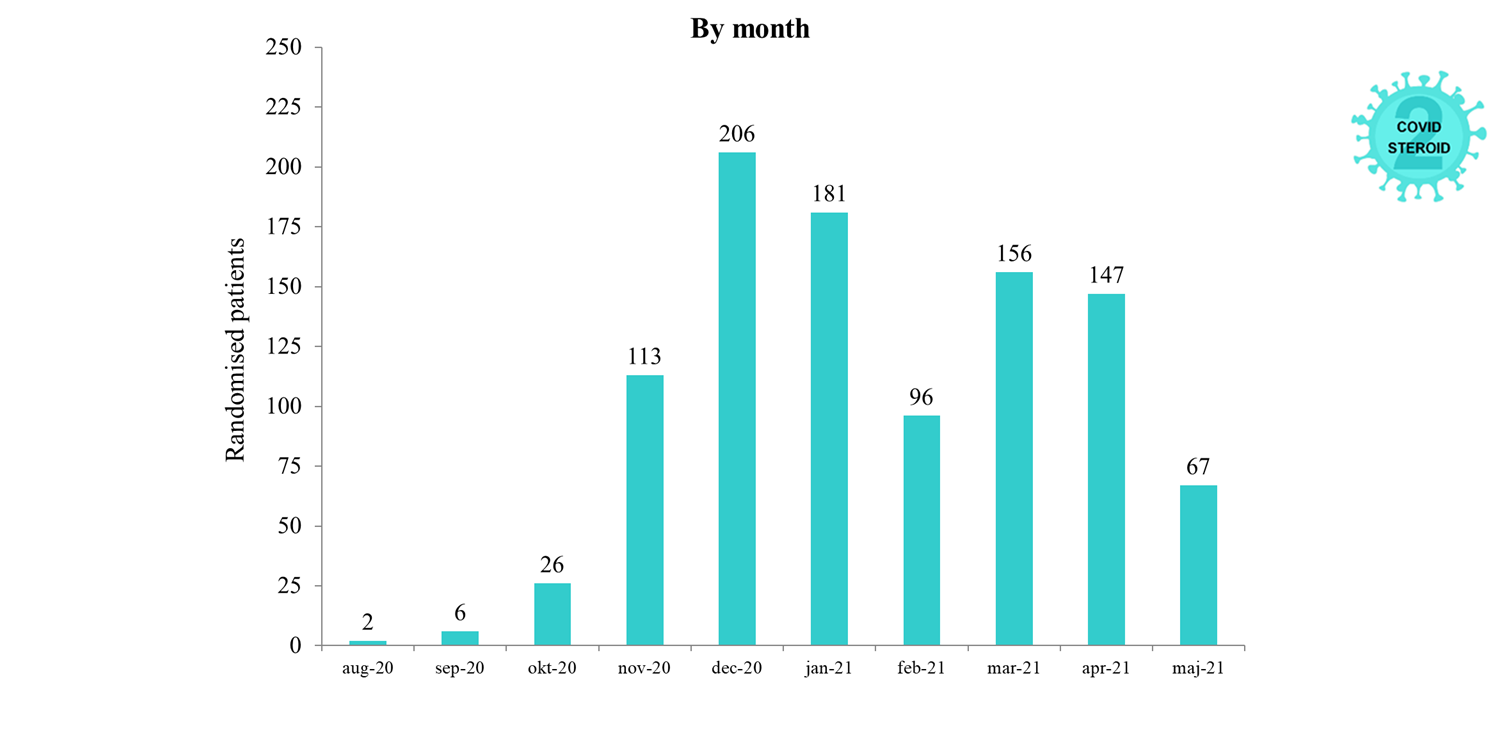
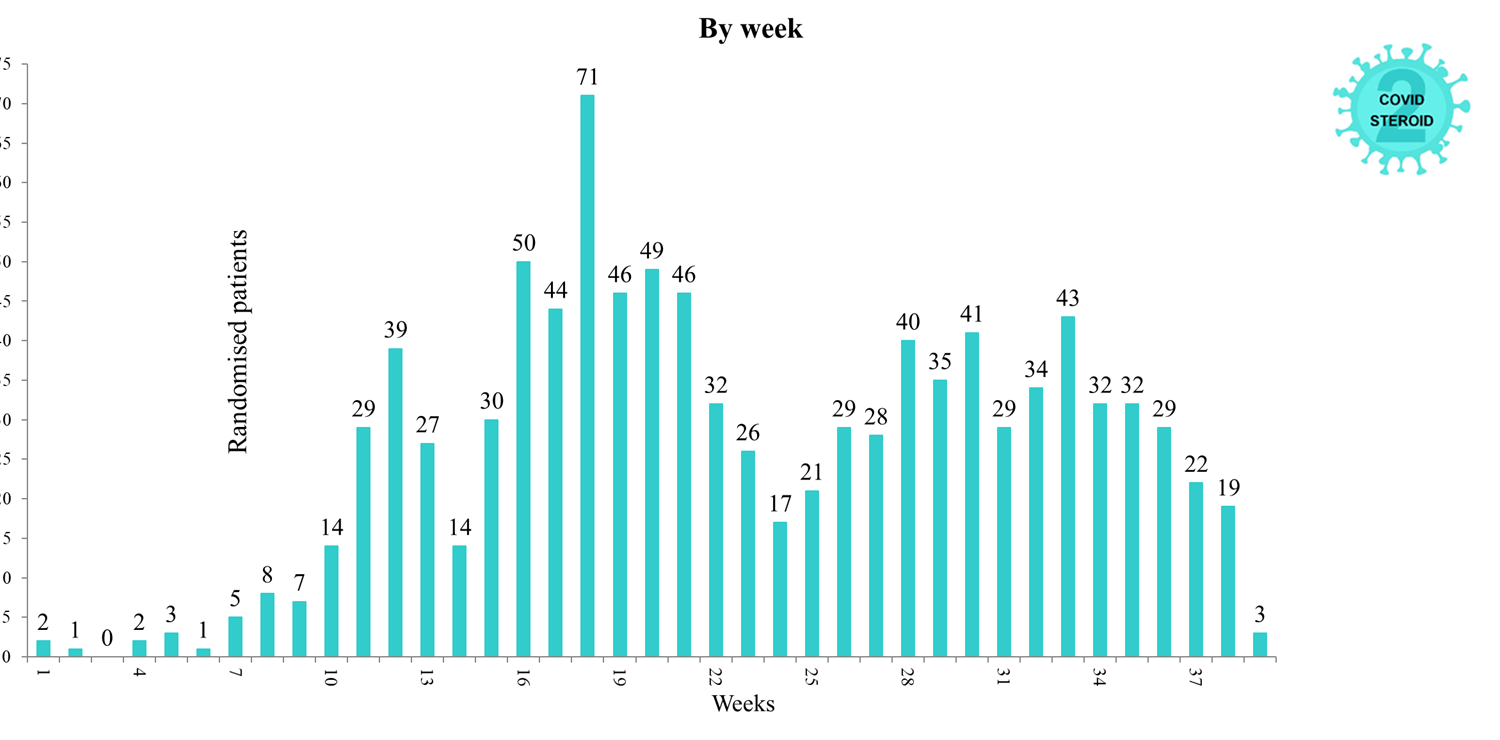
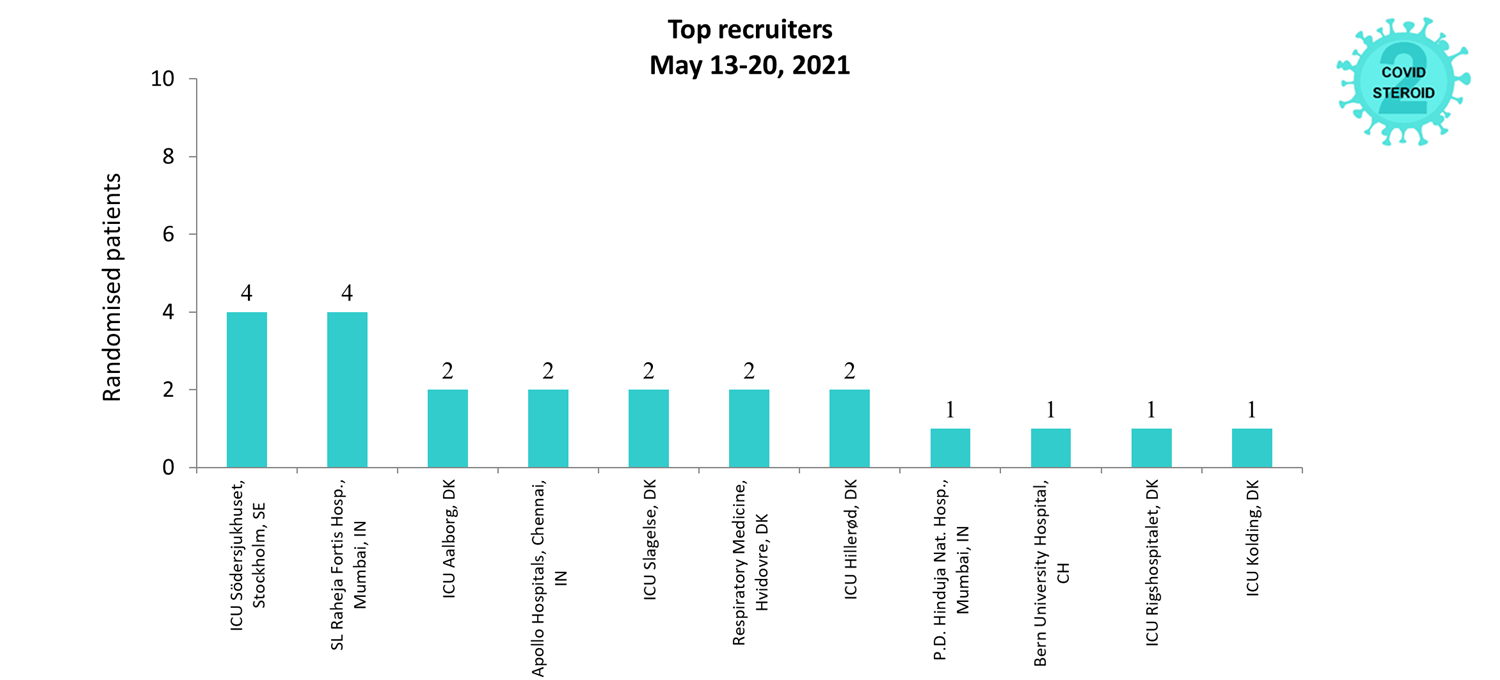
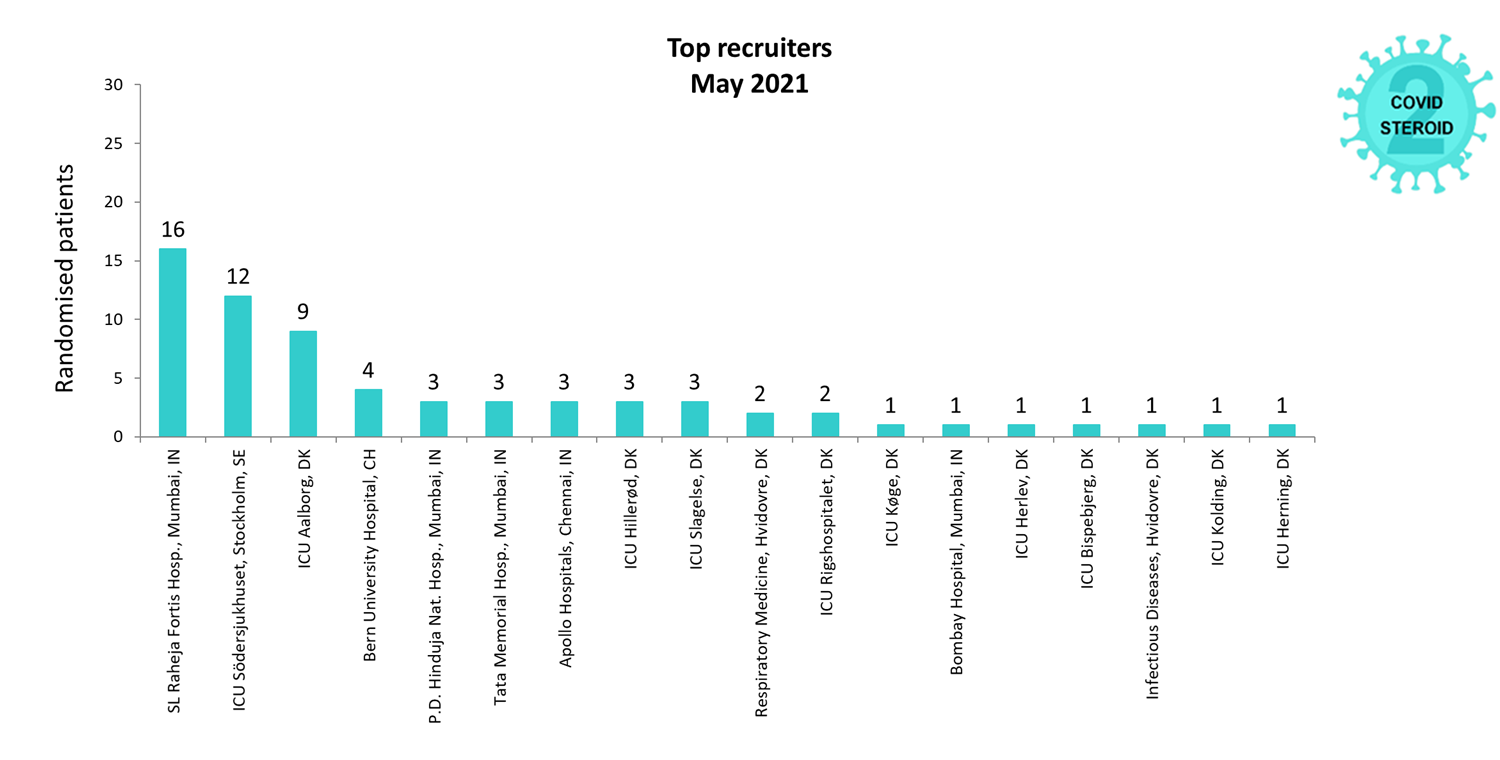
Overview of Recruitment